Atoms are the fundamental units that make up all matter in the universe. Every tangible object, from the vastness of galaxies to the smallest grain of sand, is composed of atoms. These minute entities are so small that they cannot be seen with the naked eye or even through a conventional microscope. Yet, despite their minuscule size, atoms are complex structures with a well-defined internal composition. Understanding the intricacies of atomic structure not only helps us grasp the nature of matter but also forms the foundation of chemistry and modern physics.
Table of Contents
In this comprehensive exploration, we delve into the composition of an atom, its subatomic particles, and how variations in these particles lead to the diverse elements and isotopes that populate the periodic table.
What Is an Atom?
An atom is the smallest unit of matter that retains the properties of a chemical element. It is the basic building block of matter, meaning that everything in the universe, whether solid, liquid, gas, or plasma, is made up of atoms. Atoms are indivisible by chemical means and are the smallest entities that can participate in chemical reactions. The concept of the atom has evolved over centuries, from the early philosophical musings of ancient Greek thinkers like Democritus, who first coined the term “atoms” (meaning indivisible), to the sophisticated quantum models we use today.

Atoms are incredibly small. To put this into perspective, if you were to line up 50 million hydrogen atoms side by side, the line would measure just about one centimeter. Despite their small size, atoms are not solid spheres but rather consist largely of empty space. The mass of an atom is concentrated in its nucleus, a dense region at its center, which is surrounded by a cloud of negatively charged electrons. This structure is similar to our solar system, where planets revolve around the sun, although the forces and scales involved are vastly different.
The Nucleus: The Heart of the Atom
The nucleus of an atom is the core that contains most of the atom’s mass. It is composed of two types of subatomic particles: protons and neutrons. These particles are collectively known as nucleons. The nucleus is held together by a strong nuclear force, one of the four fundamental forces of nature, which overcomes the repulsive electromagnetic force between the positively charged protons.
Protons: The Positive Charge
A proton is a subatomic particle with a positive electrical charge of +1e, where “e” represents the elementary charge. Protons are crucial in determining the identity of an element. The number of protons in the nucleus of an atom is referred to as the atomic number (denoted by the symbol Z). This number is unique to each element and defines its position in the periodic table. For example, all atoms with one proton are hydrogen atoms, while all atoms with six protons are carbon atoms.

Protons are not only carriers of positive charge but also contribute to the mass of the atom. Each proton has a mass of approximately 1 atomic mass unit (amu), which is about 1.67 × 10-27 kilograms. While this mass may seem insignificant, it is crucial in defining the overall mass of the atom.
Neutrons: The Neutral Particle
Neutrons are subatomic particles with no electrical charge, hence the name “neutron.” Like protons, neutrons have a mass close to 1 atomic mass unit, making them slightly heavier than protons but significantly heavier than electrons. Neutrons play a vital role in stabilizing the nucleus. Without neutrons, the positively charged protons would repel each other due to their like charges, causing the nucleus to fly apart. Neutrons help to mitigate this repulsion by providing an attractive force that holds the nucleus together.
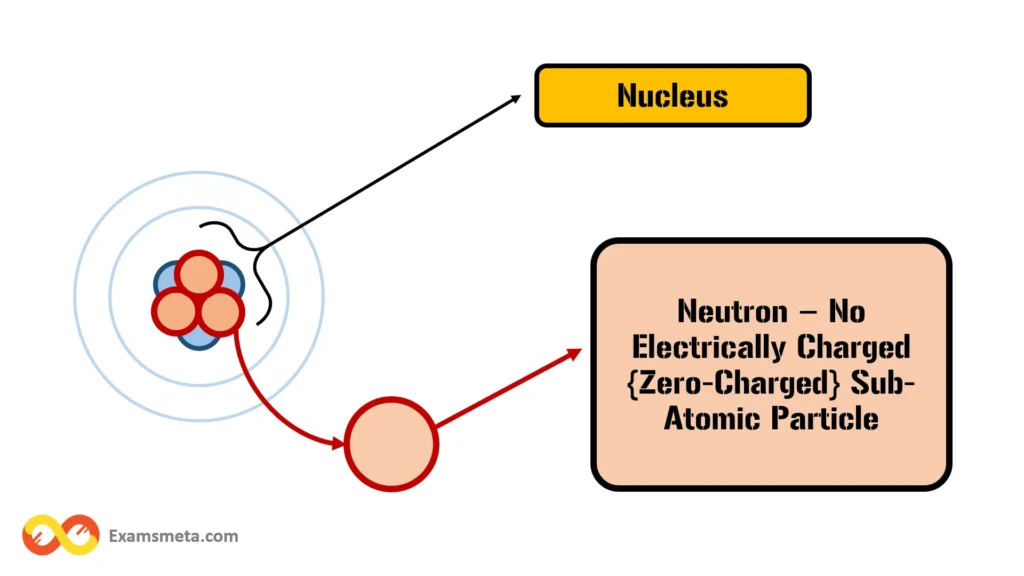
The number of neutrons in an atom can vary, even among atoms of the same element. Atoms with the same number of protons but different numbers of neutrons are known as isotopes. For example, carbon has two stable isotopes: carbon-12 (with six neutrons) and carbon-14 (with eight neutrons). The differing number of neutrons affects the atomic mass but not the chemical properties of the element.
Electrons: The Cloud Around the Nucleus
Surrounding the nucleus is a cloud of negatively charged electrons. Electrons are incredibly light, with a mass of about 1/1836 that of a proton, making them the lightest subatomic particles. Despite their small mass, electrons are essential in determining the chemical properties of an atom.

Electrons are bound to the nucleus by the electromagnetic force. Since opposite charges attract, the negatively charged electrons are drawn towards the positively charged protons in the nucleus. This attraction is what keeps the electrons in orbit around the nucleus, similar to how gravity keeps planets in orbit around the sun.
The Behavior of Electrons: Particles or Waves?
The behavior of electrons within an atom is described by the principles of quantum mechanics. Unlike classical particles, which have well-defined positions and velocities, electrons exhibit both particle-like and wave-like properties. This dual nature is a fundamental aspect of quantum mechanics and leads to the concept of orbitals—regions of space around the nucleus where there is a high probability of finding an electron.
Electrons do not orbit the nucleus in fixed paths like planets around the sun. Instead, they occupy orbitals, which are defined by complex wave functions. These orbitals come in different shapes (spherical, dumbbell-shaped, etc.) and are organized into layers known as electron shells. The arrangement of electrons in these shells and orbitals determines an atom’s chemical behavior, including how it bonds with other atoms.
Electron Configuration and Chemical Properties
The electron configuration of an atom is the distribution of electrons among the available orbitals. This configuration is crucial in determining the chemical properties of an element. For instance, the electron configuration of hydrogen is 1s¹, meaning it has one electron in its 1s orbital. In contrast, the electron configuration of carbon is 1s² 2s² 2p², indicating the presence of six electrons distributed across different orbitals.
The chemical properties of an atom are largely determined by the electrons in its outermost shell, known as valence electrons. Atoms with similar valence electron configurations tend to exhibit similar chemical behavior. For example, the elements in Group 1 of the periodic table (the alkali metals) all have a single valence electron, making them highly reactive and prone to losing that electron in chemical reactions.
Variation in the Number of Protons, Neutrons, and Electrons
While all atoms share the same basic structure of a nucleus surrounded by electrons, the number of protons, neutrons, and electrons can vary significantly between different elements and isotopes. These variations give rise to the diverse array of elements and isotopes observed in nature.
Changing the Number of Protons: Elemental Identity
The number of protons in an atom’s nucleus is the defining characteristic of an element. Changing the number of protons transforms the atom into a different element. For example, adding a proton to a hydrogen atom (which has one proton) results in a helium atom (which has two protons). This process, known as nuclear transmutation, can occur naturally in certain types of nuclear reactions or can be induced in particle accelerators.
Changing the Number of Neutrons: Isotopes
As mentioned earlier, varying the number of neutrons in an atom’s nucleus leads to the formation of isotopes. Isotopes of an element have the same number of protons (and therefore the same chemical properties) but differ in their atomic mass due to the different numbers of neutrons. Some isotopes are stable, while others are radioactive, meaning they decay over time into other elements or isotopes, releasing radiation in the process. A well-known example is carbon-14, which is used in radiocarbon dating to determine the age of ancient organic materials.
Changing the Number of Electrons: Ions
If the number of electrons in an atom changes, the atom becomes an ion. Ions are charged particles that form when an atom gains or loses electrons. An atom that loses one or more electrons becomes a cation, a positively charged ion. Conversely, an atom that gains electrons becomes an anion, a negatively charged ion. Ions are essential in many chemical processes, including the formation of salts, such as sodium chloride (NaCl), where sodium becomes a cation (Na⁺) and chlorine becomes an anion (Cl⁻).
The Quantum Mechanical Model of the Atom
The development of quantum mechanics in the early 20th century revolutionized our understanding of atomic structure. Unlike the earlier Bohr model, which depicted electrons moving in fixed orbits around the nucleus, the quantum mechanical model introduces the concept of wave-particle duality and probability distributions.
Wave-Particle Duality
In the quantum mechanical model, electrons are described not as point particles moving in fixed paths but as wave-like entities with properties of both particles and waves. This wave-particle duality means that the exact position of an electron cannot be determined with certainty. Instead, quantum mechanics provides a probability distribution that describes where an electron is likely to be found at any given time.
Orbitals and Electron Shells
The regions where electrons are likely to be found are called orbitals. Each orbital can hold a certain number of electrons, and orbitals are grouped into shells. The first shell (closest to the nucleus) has the lowest energy and can hold up to two electrons in a single s-orbital. The second shell can hold up to eight electrons, with one s-orbital and three p-orbitals. Higher shells have even more orbitals and can hold more electrons.
The distribution of electrons among these orbitals and shells determines the atom’s energy levels and influences how it interacts with other atoms. For example, when atoms absorb or emit energy, electrons can move between orbitals, leading to the phenomenon of quantum jumps or electronic transitions. These transitions are responsible for the absorption and emission spectra of atoms, which are used in spectroscopy to identify elements.
Chemical Bonding and the Role of Electrons
The arrangement of electrons in an atom’s outermost shell, or valence shell, plays a critical role in chemical bonding. Atoms bond with each other to achieve a more stable electron configuration, often by filling their valence shells to the maximum capacity.
Covalent Bonding
In covalent bonding, atoms share electrons to achieve a full valence shell. For example, two hydrogen atoms can share their single electrons to form a hydrogen molecule (H₂), where each atom has a full outer shell. Covalent bonds are strong and form the backbone of many organic molecules, including DNA, proteins, and other essential biological compounds.
Ionic Bonding
In ionic bonding, atoms transfer electrons from one to another, resulting in the formation of oppositely charged ions that attract each other. For instance, when sodium (Na) and chlorine (Cl) react, sodium donates one electron to chlorine, forming sodium ions (Na⁺) and chloride ions (Cl⁻). These ions then attract each other to form the ionic compound sodium chloride (NaCl), commonly known as table salt.
Metallic Bonding
In metallic bonding, electrons are not shared or transferred between individual atoms. Instead, they are delocalized, meaning they move freely throughout the metal lattice. This “sea of electrons” allows metals to conduct electricity and heat efficiently and gives them their characteristic malleability and ductility.
The Periodic Table: Organizing the Elements
The periodic table is a tabular arrangement of elements based on their atomic number, electron configuration, and recurring chemical properties. The table is organized into periods (rows) and groups (columns), with elements in the same group sharing similar chemical properties due to their similar valence electron configurations.
Trends in the Periodic Table
Several important trends can be observed in the periodic table:
- Atomic Radius: As you move down a group, the atomic radius increases due to the addition of electron shells. As you move across a period, the atomic radius decreases due to the increasing nuclear charge pulling the electrons closer to the nucleus.
- Ionization Energy: The energy required to remove an electron from an atom increases across a period (from left to right) and decreases down a group.
- Electronegativity: The tendency of an atom to attract electrons in a chemical bond increases across a period and decreases down a group.
The Role of Electron Configuration in Chemical Behavior
The periodic table’s structure reflects the underlying electron configuration of the elements. For example, the noble gases in Group 18 have full valence shells, making them chemically inert. In contrast, the alkali metals in Group 1 have a single valence electron, making them highly reactive and eager to lose that electron in chemical reactions.
Conclusion: The Atom is the Fundamental Unit of Matter
The atom, with its nucleus of protons and neutrons surrounded by a cloud of electrons, is the fundamental building block of matter. Understanding the composition of the atom, the behavior of its subatomic particles, and how these particles interact to form elements and compounds is essential to the study of chemistry and physics.
From the tiny hydrogen atom with its single proton to the complex uranium atom with 92 protons and numerous isotopes, the diversity of elements in the universe arises from the simple yet profound variations in the number of protons, neutrons, and electrons. These variations give rise to the chemical properties that govern everything from the air we breathe to the stars that light our sky. The study of atoms is not just a study of matter but a journey into the very essence of the universe itself.
Informative Table Based on the Composition of an Atom
Understanding the composition of an atom is fundamental to grasping the principles of chemistry and physics. The following table provides a detailed breakdown of the various components of an atom, their properties, and their significance. This comprehensive table is designed to serve as a quick reference for anyone looking to explore the intricate details of atomic structure, subatomic particles, and the behavior of atoms in different chemical contexts.
| Aspect | Details |
|---|---|
| Definition of an Atom | An atom is the smallest unit of matter that retains the properties of a chemical element. It is indivisible by chemical means and serves as the fundamental building block of all matter. |
| Size and Structure | Atoms are extremely small, with most of their space being empty. The mass of an atom is concentrated in the nucleus, which is surrounded by a cloud of electrons. Despite their size, atoms have a complex internal structure, akin to a miniature solar system, where electrons orbit the dense nucleus. |
| Nucleus | The nucleus is the core of the atom, containing most of its mass. It consists of protons and neutrons, collectively known as nucleons. The nucleus is held together by the strong nuclear force, overcoming the repulsive force between positively charged protons. |
| Protons | Protons are positively charged subatomic particles found in the nucleus. They have a charge of +1e and a mass of approximately 1 atomic mass unit (amu). The number of protons in an atom, known as the atomic number (Z), determines the element’s identity. For example, hydrogen has one proton, while carbon has six. |
| Neutrons | Neutrons are neutral subatomic particles that reside in the nucleus alongside protons. They have no electrical charge but contribute significantly to the atom’s mass (approximately 1 amu). Neutrons stabilize the nucleus by mitigating the repulsive force between protons. The number of neutrons can vary within the same element, leading to the formation of isotopes. |
| Electrons | Electrons are negatively charged subatomic particles that orbit the nucleus. They have a mass of about 1/1836 that of a proton, making them extremely light. Electrons determine an atom’s chemical properties and are involved in chemical bonding. The behavior of electrons is described by quantum mechanics, where they exhibit both particle-like and wave-like properties. |
| Electron Behavior | Electrons exhibit wave-particle duality and occupy regions around the nucleus known as orbitals. These orbitals are probability distributions where electrons are likely to be found. The arrangement of electrons in these orbitals and electron shells determines the atom’s chemical behavior, including its ability to bond with other atoms. |
| Electron Configuration | The electron configuration describes the distribution of electrons in an atom’s orbitals. This configuration determines the atom’s energy levels and chemical properties. For instance, hydrogen has an electron configuration of 1s¹, while carbon’s configuration is 1s² 2s² 2p². The outermost electrons, known as valence electrons, play a crucial role in chemical bonding. |
| Chemical Bonding | Chemical bonding occurs when atoms interact to achieve more stable electron configurations. Covalent bonding involves the sharing of electrons between atoms, while ionic bonding involves the transfer of electrons, resulting in oppositely charged ions that attract each other. Metallic bonding features delocalized electrons that move freely throughout a metal lattice. |
| Periodic Table | The periodic table organizes elements based on their atomic number, electron configuration, and recurring chemical properties. Elements in the same group (column) have similar valence electron configurations, leading to similar chemical behaviors. Key trends include variations in atomic radius, ionization energy, and electronegativity across periods (rows) and groups (columns). |
| Atomic Number (Z) | The atomic number (Z) is the number of protons in an atom’s nucleus, defining the element’s identity. It increases across the periodic table, leading to a corresponding increase in the number of electrons and often the number of neutrons, though the neutron count can vary among isotopes. |
| Isotopes | Isotopes are atoms of the same element that have different numbers of neutrons. While they share the same atomic number (protons), they have different atomic masses due to the variation in neutron count. Some isotopes are stable, while others are radioactive and decay over time, emitting radiation and transforming into other elements or isotopes. |
| Ions | Ions are charged atoms formed when an atom gains or loses electrons. Cations are positively charged ions that form when an atom loses electrons, while anions are negatively charged ions that form when an atom gains electrons. Ions are critical in various chemical reactions, particularly in the formation of salts and other ionic compounds. |
| Wave-Particle Duality | Wave-particle duality is a key concept in quantum mechanics, describing how subatomic particles like electrons exhibit both wave-like and particle-like properties. This duality is fundamental to understanding the behavior of electrons within atoms, especially in how they occupy orbitals and interact with other particles. |
| Orbitals and Electron Shells | Protons are positively charged subatomic particles found in the nucleus. They have a charge of +1e and a mass of approximately 1 atomic mass unit (AMU). The number of protons in an atom, known as the atomic number (Z), determines the element’s identity. For example, hydrogen has one proton, while carbon has six. |
| Quantum Jumps | Quantum jumps or electronic transitions occur when electrons move between orbitals of different energy levels. This movement can absorb or emit energy in the form of light, leading to the characteristic absorption and emission spectra of elements. These spectra are used in spectroscopy to identify elements and study their properties. |
| Nuclear Transmutation | Nuclear transmutation refers to the process of changing one element into another by altering the number of protons in the nucleus. This can occur naturally in certain types of nuclear reactions, such as radioactive decay, or can be induced artificially in particle accelerators. An example is the conversion of nitrogen into oxygen through the capture of an additional proton. |
| Valence Electrons | Valence electrons are the electrons in the outermost shell of an atom. These electrons are primarily responsible for the chemical properties of the atom, including its reactivity and the types of bonds it can form. Elements with similar valence electron configurations, such as those in the same group of the periodic table, tend to exhibit similar chemical behaviors. |
| Covalent Bonding | In covalent bonding, atoms share pairs of electrons to achieve a full outer shell and stabilize themselves. This type of bond is strong and forms the backbone of many biological molecules, such as DNA and proteins. Covalent bonds are common in organic chemistry and are crucial for the structure and function of living organisms. |
| Ionic Bonding | Ionic bonding involves the transfer of electrons from one atom to another, resulting in the formation of oppositely charged ions. These ions attract each other and form ionic compounds, such as sodium chloride (NaCl). Ionic bonds are typically found in salts and other compounds where the difference in electronegativity between the bonding atoms is significant. |
| Metallic Bonding | Metallic bonding is characterized by a “sea of electrons” that are free to move throughout a metal’s lattice structure. This type of bonding gives metals their unique properties, such as electrical conductivity, malleability, and ductility. In metallic bonds, electrons are not associated with individual atoms but are instead shared collectively across the entire structure. |
| Spectroscopy | Spectroscopy is a technique used to study the interaction between matter and electromagnetic radiation. In the context of atomic structure, spectroscopy involves analyzing the light absorbed or emitted by electrons as they transition between energy levels. This analysis provides valuable information about the composition, structure, and behavior of atoms and molecules. |
| Radioactive Decay | Radioactive decay is the process by which unstable isotopes (radioisotopes) lose energy by emitting radiation, transforming into a different element or isotope in the process. This decay occurs at a predictable rate, known as the half-life, and is the basis for techniques such as radiocarbon dating. Radioactive decay plays a crucial role in nuclear chemistry and astrophysics. |
| Periodic Trends | The periodic table reveals several trends that help predict the properties of elements. For example, atomic radius generally increases down a group and decreases across a period, while ionization energy and electronegativity typically increase across a period and decrease down a group. These trends are due to the underlying electron configurations and nuclear charge of the elements. |
| Noble Gases | The noble gases are a group of elements in Group 18 of the periodic table. They are characterized by having a full valence |
Related Article
- Comprehensive Guide to Stoichiometry: Concepts, Calculations, & Real-Life Applications
- Comprehensive Guide to Percentage Composition in Chemistry
- Understanding the Mole Concept: A Comprehensive Guide
- Gram Atomic Mass and Gram Molecular Mass: A Comprehensive Guide
- Dalton’s Atomic Theory: An Interesting Groundbreaking Scientific Concept
- Laws of Chemical Combination: The Foundations of Chemical Reactions
- Understanding Measurement Uncertainty in Chemistry with Perfect Explanation
- Properties of Matter: An In-Depth Exploration
- Understanding Matter: States, Properties, and Beyond
- The Importance of Chemistry in Everyday Life
Frequently Asked Questions (FAQs)
What is an atom?
An atom is the smallest unit of matter that retains the properties of a chemical element. It is the basic building block of matter and cannot be divided without releasing electrically charged particles. An atom consists of a central nucleus surrounded by a cloud of electrons.
What makes up the nucleus of an atom?
The nucleus of an atom is made up of protons and neutrons. Protons carry a positive charge, while neutrons have no charge (they are neutral). The nucleus is extremely dense and contains more than 99.94% of the atom’s total mass.
How are electrons arranged in an atom?
Electrons are negatively charged particles that orbit the nucleus of an atom in regions called orbitals. These orbitals are part of larger groupings known as shells. The arrangement of electrons in these orbitals and shells determines the chemical properties of the atom.
What are the differences between protons, electrons, and neutrons?
- Protons are positively charged particles found in the nucleus, with a mass slightly less than that of a neutron.
- Electrons are negatively charged particles that orbit the nucleus and have a much smaller mass than protons or neutrons.
- Neutrons are neutral particles found in the nucleus with a mass similar to that of a proton.
What role do protons play in an atom?
Protons determine the identity of an atom. The number of protons in the nucleus, known as the atomic number, defines the element. For example, an atom with one proton is hydrogen, while an atom with six protons is carbon.
What happens if the number of protons in an atom changes?
If the number of protons in an atom changes, the atom becomes a different element. For instance, if a carbon atom (with six protons) gains one proton, it becomes a nitrogen atom (with seven protons).
What is an electron and what is its significance in an atom?
An electron is a subatomic particle with a negative charge. Electrons are fundamental to the atom’s chemical behavior because they are involved in forming bonds with other atoms. Their arrangement in orbitals and shells around the nucleus influences how the atom interacts with others.
How does the number of electrons affect an atom?
The number of electrons in an atom determines its charge. If the atom has more electrons than protons, it becomes a negatively charged ion. If it has fewer electrons than protons, it becomes a positively charged ion.
What is a neutron and why is it important?
A neutron is a neutral particle found in the nucleus of an atom. Neutrons contribute to the mass of the atom and play a crucial role in the stability of the nucleus. The number of neutrons can vary in atoms of the same element, leading to different isotopes.
What are isotopes?
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon, with 6 protons but 6 and 8 neutrons, respectively. Isotopes have different masses and may exhibit different physical properties.
How does the structure of an atom compare to the solar system?
The structure of an atom is often compared to the solar system. In this analogy, the nucleus is like the sun, containing most of the atom’s mass, while the electrons are like planets, revolving around the nucleus in defined orbits. However, unlike planets, the nucleus is extremely small compared to the entire atom, and electrons do not follow fixed orbits but exist in regions of probability known as orbitals.
What is the significance of quantum mechanics in understanding atoms?
Quantum mechanics is crucial for understanding the behavior of electrons in an atom. It provides a framework for explaining how electrons exist in specific orbitals and how these orbitals determine the chemical properties of the atom. Quantum mechanics reveals that electrons exhibit both particle-like and wave-like behavior.
What is the atomic number and why is it important?
The atomic number is the number of protons in the nucleus of an atom. It is a fundamental property that determines the identity of an element and its position in the periodic table. The atomic number also indicates the number of electrons in a neutral atom, which influences its chemical behavior.
How does the periodic table relate to the structure of an atom?
The periodic table arranges elements in order of increasing atomic number (number of protons). As you move across the table, each element has more protons, neutrons, and electrons than the one before it. This arrangement reflects periodic trends in the properties of elements, which are influenced by the structure of their atoms.
What happens if the number of neutrons in an atom changes?
If the number of neutrons in an atom changes, it forms a different isotope of the same element. Isotopes have the same number of protons and electrons, so they retain the chemical properties of the element, but their physical properties, such as mass and stability, may differ.
Why can’t atoms be seen with the naked eye or a normal microscope?
Atoms are incredibly small, with sizes on the order of angstroms (1 angstrom = 10^-10 meters). This scale is far smaller than the wavelength of visible light, which is why atoms cannot be seen with the naked eye or even with a normal optical microscope. To visualize atoms, advanced techniques such as scanning tunneling microscopy (STM) or atomic force microscopy (AFM) are required.
What is the relationship between electrons and chemical bonding?
Electrons play a crucial role in chemical bonding. Atoms bond with each other by either sharing or transferring electrons, which results in the formation of molecules. There are different types of bonds, such as covalent bonds (where electrons are shared) and ionic bonds (where electrons are transferred), and the arrangement of electrons in an atom’s outer shell determines how it will bond with other atoms.
How does the concept of orbitals differ from the traditional idea of orbits?
In traditional models, electrons were thought to revolve around the nucleus in fixed paths, similar to planets around the sun. However, quantum mechanics introduced the concept of orbitals, which are regions of space where there is a high probability of finding an electron. Unlike fixed orbits, orbitals do not have a precise path; instead, they represent the statistical distribution of where an electron is likely to be found.
What is an ion, and how is it formed?
An ion is an atom or molecule that has gained or lost one or more electrons, giving it a net electrical charge. If an atom loses electrons, it becomes a positively charged cation; if it gains electrons, it becomes a negatively charged anion. Ions are formed through chemical reactions, such as when sodium loses an electron to become Na⁺ or chlorine gains an electron to become Cl⁻.
Why is the mass of an atom concentrated in the nucleus?
The mass of an atom is concentrated in the nucleus because the nucleus contains protons and neutrons, which are much more massive than electrons. While electrons contribute to the overall structure and size of the atom, their mass is negligible compared to that of the nucleons (protons and neutrons), which together account for nearly all of the atom’s mass.
What is the difference between an atom and a molecule?
An atom is the smallest unit of an element that retains the chemical properties of that element. It consists of a nucleus, containing protons and neutrons, surrounded by electrons in various orbitals. A molecule is a group of two or more atoms that are chemically bonded together. These bonds can be covalent, where atoms share electrons, or ionic, where electrons are transferred from one atom to another, resulting in the formation of oppositely charged ions that attract each other. For example, a water molecule (H₂O) consists of two hydrogen atoms covalently bonded to one oxygen atom.
What are isotopes, and how do they differ from one another?
Isotopes are different forms of the same element that contain the same number of protons but different numbers of neutrons in their nuclei. This difference in neutron count results in different atomic masses for the isotopes. For instance, carbon has three naturally occurring isotopes: carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C). While ¹²C and ¹³C are stable, ¹⁴C is radioactive and decays over time, which is used in carbon dating to determine the age of ancient objects.
What is the periodic table and why is it important?
The periodic table is a systematic arrangement of all known chemical elements in order of increasing atomic number (the number of protons in an atom’s nucleus). Elements with similar chemical properties are grouped together in columns known as groups or families. The table is important because it organizes elements in a way that reveals patterns in their properties, such as ionization energy, electronegativity, and atomic radius. This organization allows chemists to predict the behavior of elements and their compounds, making it a fundamental tool in the study and application of chemistry.
How do chemical reactions occur, and what is the role of a catalyst?
Chemical reactions occur when substances (reactants) undergo a process that leads to the formation of new substances (products) with different chemical properties. This process involves the breaking of old bonds and the formation of new ones. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. It works by lowering the activation energy needed for the reaction to proceed, making it easier for the reactants to convert into products. For example, in the Haber process for ammonia production, an iron catalyst is used to accelerate the reaction between nitrogen and hydrogen gases.
What is pH and how does it relate to acidity and basicity?
pH is a scale used to measure the acidity or basicity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration ([H⁺]) in a solution. The pH scale ranges from 0 to 14, with 7 being neutral. A pH value below 7 indicates an acidic solution, where the concentration of hydrogen ions is higher than that of hydroxide ions ([OH⁻]). A pH value above 7 indicates a basic (or alkaline) solution, where hydroxide ions outnumber hydrogen ions. The pH of a solution is crucial in many chemical and biological processes, including enzyme activity and cellular function.
What are intermolecular forces and why are they important?
Intermolecular forces are forces of attraction between molecules, as opposed to the forces within a molecule (intramolecular forces like covalent or ionic bonds). These forces include dipole-dipole interactions, hydrogen bonding, and London dispersion forces. They are weaker than chemical bonds but are critical in determining the physical properties of substances, such as boiling points, melting points, viscosity, and solubility. For instance, the relatively high boiling point of water is due to strong hydrogen bonds between water molecules.
What is the significance of electron configuration in understanding an element’s properties?
Electron configuration refers to the arrangement of electrons in an atom’s orbitals. This configuration determines how an atom interacts with other atoms, influencing its chemical properties such as reactivity, ionization energy, and electronegativity. The valence electrons (the electrons in the outermost shell) are particularly important, as they are involved in forming chemical bonds. For example, the electron configuration of sodium (Na) is [Ne]3s¹, indicating that it has one valence electron that it can easily lose to form a positive ion (Na⁺), making it highly reactive.
What are acids and bases, and how do they react with each other?
Acids are substances that donate protons (H⁺ ions) in a solution, while bases are substances that accept protons. When an acid and a base react together, they typically undergo a neutralization reaction, producing salt and water. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are sodium chloride (NaCl) and water (H₂O). This reaction can be represented as HCl + NaOH → NaCl + H₂O. The strength of an acid or base is determined by its ability to donate or accept protons, which is reflected in its pH value.
How does the concept of hybridization explain the shapes of molecules?
Hybridization is a concept in chemistry that describes the mixing of atomic orbitals to form new hybrid orbitals. These hybrid orbitals have shapes and energies that are different from the original atomic orbitals and are better suited for bonding in molecules. For example, in methane (CH₄), the carbon atom undergoes sp³ hybridization, where one s orbital and three p orbitals combine to form four equivalent sp³ hybrid orbitals. These orbitals arrange themselves in a tetrahedral geometry around the carbon atom, resulting in the characteristic shape of the methane molecule.
What is the role of Gibbs free energy (ΔG) in predicting the spontaneity of a chemical reaction?
Gibbs free energy (ΔG) is a thermodynamic quantity that combines the concepts of enthalpy (ΔH) and entropy (ΔS) to predict whether a chemical reaction will occur spontaneously. The equation ΔG = ΔH – TΔS (where T is the temperature in Kelvin) is used to determine the spontaneity of a reaction. A negative ΔG indicates that a reaction is spontaneous, meaning it can occur without any additional input of energy. Conversely, a positive ΔG means the reaction is non-spontaneous and requires energy to proceed. Understanding ΔG is crucial in both industrial processes and biological systems, where controlling reaction spontaneity is essential for efficiency and function.
